Glaucoma is best defined as death of the optic nerve head (ONH) or more technically, progressive optic neuropathy. The optic nerve is the nerve that travels into the back of the eye and goes across the retinal surface. The ONH allows the signals from the retina to be taken back to the brain.
There are many types of Glaucoma and are classified depending on whether the anterior chamber angle is open or closed. Glaucoma can occur on it’s own (primary) or can occur as a result of another ocular or systemic condition (secondary).
Aqueous Pathway
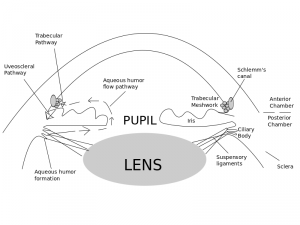
To understand the different types of Glaucoma we must know the aqueous pathway. Aqueous is a liquid that supports and provides nutrition for the eye (especially the lens and cornea). Aqueous also forms the anterior chamber.
Aqueous is produced in the ciliary body behind the Iris. This liquid then travels in the posterior chamber, through the pupil and then spreads out in the anterior chamber. 90% of this fluid then drains back into the circulation of the eye through the trabecular meshwork (which acts as a sieve/filter) and then the canal of schlemm (which is like a small drain). The other 10% exits the eye via the venous circulation of the ciliary body, choroid and sclera. A tiny amount also drains through the Iris.
Types of Glaucoma
Primary Open Angle Glaucoma (POAG)
This normally occurs when there is a dysfunction of the trabecular meshwork.
Risk Factors include
- High IOP
- Age
- Race (afro-caribbean at a greater risk than Caucasian)
- Family History
- Thin corneas
- Other systemic diseases (e.g diabetes)
- Myopia
- ONH asymmetry
How to screen?
Typically in the UK, referral is based upon 3 assessments/findings
- Elevated IOP
- ONH changes
- Visual field (VF) changes
CHECKING IOP
You can use contact (applanation) or non contact (non applanation) technique. Applanation is considered to be the most accurate. Methods include Goldman (gold standard) or Icare (also very good). There are other methods such as the tonopen.
Method for Goldman Contact Tonometry
Goldman contact tonometry works by measuring how much force it takes to flatten a certain area of the cornea
- Instill 1% drop of an anaesthetic and flouroscein combination (eg. proximetacaine+flouroscein)
- Take either a disposible probe or reusable probe (once cleaned) and place in Goldman
- Turn on blue light of the slit lamp
- Ask the patient to pop their chin on the rest and their forehead touching the bar above
- Line up the slit lamp and Goldman and instruct the patient to look straight ahead
- Move the slit lamp forward until the probe makes contact with the cornea
- Semi rings will then be seen. Use the dial on the side of the Goldman to adjust the pressure of the probe on the eye
- You want the inner part of each semi ring to be touching. Then you have achieved the correct pressure in the eye. If the rings overlap, the corneal pressure reads too high and the cornea has been flattened too much. If the rings are far apart, the corneal reading is too low and the cornea is flattened too little (need a diagram here)
Guidelines are as follows:
For patients with ocular hypertension or suspected glaucoma a reliable baseline measure of intraocular pressure is required. A minimum of two intraocular pressure readings on a single occasion using the same tonometer is recommended. The type of tonometer and the time of measurement should be specified in any referral to secondary-eye-care services. (sign guidelines March 2015)
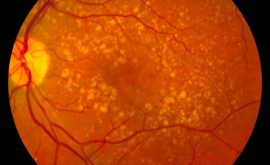
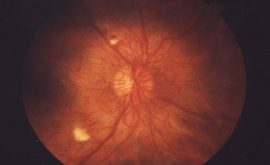
OPTIC NERVE CHANGES
- Disc size, PPA cup to disc ratio and disc/rim ratio
- Neural retinal rim changes
- Vessel changes
- Lamina Cribosa presence and changes
- Retinal nerve fibre layer changes
When examining the ONH try and be systematic, The above list is the order in which I examine and assess the ONH.
1 DISC SIZE AND PPA
It is possible to measure the ONH size using the slit lamp and a condensing (volk) lens. The slit beam height can be adjusted when viewing the fundus through a volk lens. A graticule gives the size in mm. An adjustment must then be made depending on which volk lens was used.
Table 1. Correction factor for Volk Lens used with the Haag-Streit slit lamp (10x magnification)
| Volk lens type | Correction Factor |
| 60D | 0.9 |
| 78D | 1.1 |
| 90D | 1.3 |
| Superfield | 1.5 |
The disc height is becoming increasingly important as recent guidelines have recommended that we measure the size of the optic disc and the ratio of the narrowest rim/disc be assessed. The guidelines for referral to secondary eye care services are presented below:
• small discs (<1.5 mm in diameter) where the narrowest rim/disc ratio is <0.3
• medium discs (1.5–2.0 mm in diameter) where the narrowest rim/disc ratio is <0.2
• large discs (>2.0 mm in diameter) where the narrowest rim/ disc ratio is <0.1
For full reference http://sign.ac.uk/pdf/SIGN144.pdf
Cup to disc ratio is important as a large cup can be a sign of Glaucoma. However, many normal eyes have large cups (physiological cupping) and careful examination and consideration of all findings need to be done prior to referral.
Para-papillary chorioretinal atrophy (PPA) consists of irregularities of the retinal tissues surrounding some or all of the disc margin. The underlying choroid is completely intact, and therefore PPA differs from myopic scleral crescents or tilted disc scleral crescents. It is very common and present in almost all normal eyes. There are two distinct sub-types;
- Βeta zone (β PPA) – pale zone around the peripapillary scleral ring and is characterized by visible sclera and large choroidal blood vessels.
- Alpha zone (α PPA) – variable irregular pigmentation of the RPE. On its outer edge it is adjacent to normal retinal tissue, with the inner edge either next to beta PPA, or the peripapillary scleral ring if no β PPA is present
PPA can be a sign of damage to the ONH. Normally, when there are larger zones of both alpha and beta PPA with beta PPA being present more frequently. There is also evidence to suggest that the area with greatest PPA correlates to the area of glaucomatous ONH damage. Change in PPA does not appear to occur in non-glaucomatous optic neuropathies.
2 NEURAL RETINAL RIM
The neuroretinal rim (NRR) is the nerve tissue between the disc margin and the outer edge of the cup. When there is damage to the ONH, initial changes may be to the NRR. We commonly assess the rim using the ISNT rule. This is where the inferior rim is thickest, superior next thickest, nasal next thickest and temporal thinnest. If this rule is broken, the optic nerve may be suspicious. As previously mentioned, new guidelines have mentioned that both the NRR and rim/disc ratio need to be considered.
3 VESSEL CHANGES
These are normally seen a little later but include:
- narrowing of vessels – these can be hard to see but there is evidence to suggest that focal narrowing of up to 50% can be seen with ONH damage
- Baring of circumlinear vessels – These vessels are small arteriole or venules that sit upon neural tissue and have a route temporally across the disc towards the macula region. NRR damage leaves the vessels with no supportive tissue making them look out of place. They are also known as ‘flyover’ vessels
- Bayonetting – Vessels that move nasally across the cup base can indicate degrees of glaucomatous NRR loss that has resulted in the remaining NRR being undercut by the pathological excavation. Because these blood vessels travel up the temporal cup face, they will disappear from view when the NRR becomes severely undermined, producing a mismatch between their position at the cup base and on the remaining NRR. This appearance is descriptively termed “bayonet sign” due to characteristic misaligned appearance, comparable to a bayonet fixing upon a rifle.
- Nasalisation of blood vessels – Physiologically the central retinal artery and vein move through the lamina cribrosa at a position close the disc centre, the central retinal artery usually being more nasal than the vein. Both make a sub division into superior and inferior branch vessels soon after the point at which they are first visible. In the majority of normal eyes, these branch vessels move up the superior and inferior aspects of the nasal cup face towards the retinal plane. More nasal location of the larger branch vessels can be seen as the cup expands pathologically in GON as supportive nasal NRR becomes reduced in breadth.
4 LAMINA CRIBOSA CHANGES
The lamina cribosa is visible when the disc is deep. Look for changes in the pores of the lamina cribosa. If they are round, they are normal, slightly oval may indicate changes and slit like is suspicious.
5 RETINAL NERVE FIBRE
The retinal nerve fibre layer (RNFL) is a layer of striations (fibre bundles) that run from the optic nerve towards the periphery. The striations look like silver/white threads and are best viewed with a bright, red-free light (green filter). It is easier to see in eyes with heavily pigmented retinal pigment epithelium (RPE) and choroid and may be very hard to see in eyes with blonde fundii. The RNFL is most visible within 2 disc diameters (DD) of the ONH and is brightest infero and supero-temporally, where the fibre bundles are most dense. Age and media opacities also make it harder to see.
We need to look for changes in this RNFL. The changes can be focal, localized or diffuse. If clearly seen, it can be a significant sign of glaucomatous optic nerve head changes. If a correlation is made with a visual field defect it is even more valuable.
Localised
- Slit, or wedge shaped defects which are within 2 DD of the disc margin and wider than retinal vessels.
- Surrounding area appears darker and has a matt appearance
VISUAL FIELD
Glaucoma causes visual defects of particular patterns. Examples include an enlarged blind spot, nasal step and arcuate defect.
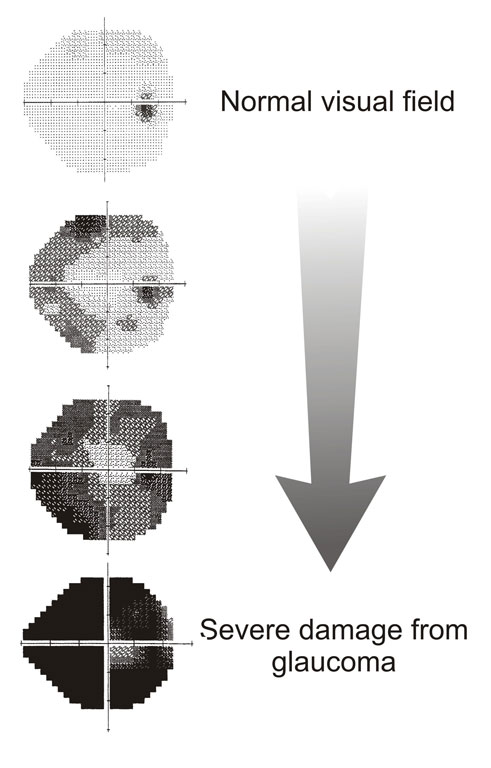
NOTE: A detailed article on visual field defects will follow
CENTRAL CORNEAL THICKNESS
To measure the corneal thickness, an instrument called a Pachymeter is used. This device uses ultrasound to measure how thick the cornea is. Patients who have thinner than average corneas have artificially lower IOP readings and patients who have thicker corneas have artificially higher IOP readings. It is important to check this and adjust IOP readings accordingly. There is some evidence that suggests patients with thin corneas may increase their chances of having Glaucoma.
Closed Angle Glaucoma (CAG)
This type of Glaucoma occurs due to closure or obstruction of the anterior chamber angle. In primary angle closure the mechanism as to why this closure occurs is poorly understood but what is known is that there is a shift of pressure where the posterior chamber pressure exceeds that of the anterior chamber due to the pupil not allowing the aqueous to sufficiently enter the anterior chamber (pupillary block). As a result, the iris and lens move forward and tilt up, closing the angle. This angle closure causes the pressure in the eye to increase very fast and can severely damage the ONH if not treated quickly.
Risk Factors
- shallow a/c
- small eyes
- Asian ethnicity
- large cataracts
- pupil dilation
Checking the anterior chamber depth
- Gross test using pen light – shine a pen light side on across cornea. The whole iris should light up, if there are any areas of shadowing – this may indicate a shallow angle.
- Van herick’s technique – using a slit lamp, shine a very thin and bright slit beam on the peripheral (nasal and temporal) cornea. Check the ratio of the peripheral anterior chamber width compared to the corneal width. Refer for risk of angle closure if ratio is less than 1:4/grade 1 or below. diagram to explain needed
- Gonioscopy – this is the only way to actually see the angle. A Gonio lens is a lens which has one or more glass mirrors. The glass mirror allows light to travel forward, counter acting internal reflection and allowing the angle to be seen. It is a difficult technique to master but is very valuable and worth spending time on. (another article will focus on this and go through technique)
It’s worth mentioning that there are many sub groups of CAG.
These include intermittent (or subacute), acute, post-congestive and chronic angle closure.
- intermittent/sub acute – CAG may be due to pupil dilation where the a/c angle gets crowded when the iris dilates. This may occur in a dark room when watching T.V. When the pupil then constricts, the pupillary body is spontaneously relieved, re opening the angle and lowering the IOP.
- Acute – this is a sight threatening emergency. The a/c angle is completely blocked and the patient presents with a red, painful eye. They may feel nauseous and the pupil will be mid dilated. The patient will complain about halos around lights and the cornea will appear hazy.
- post congestive angle closure – this happens after an acute attack and is due to associated trabecular or ciliary body damage post attack/treatment.
- Chronic angle closure – This occurs when the angle gradually closes over time due to anteriorly located ciliary processes. It can also be caused as a result of many intermittent attacks or if the patient has POAG in conjunction with narrow angles and has been taking miotic drops for a sustained period of time.
In depth detail of these subsections are outwith the scope of this article.
Treatment of Glaucoma
Medical or Surgical?
The goal is to either decrease aqueous production, increase aqueous outflow or both.
Medical treatment
- Mainly for open angle glaucoma
Decrease production – beta blockers and CAIs
Increase outflow – prostaglandin analogues and alpha agonists
Surgical Treatment
- For closed angle and open angle glaucoma
Selective Laser Therapy – A YAG laser is used to selectively laser pigmented TM cells without inducing photocoagulation and collateral damage to non-pigmented cells, This is achievable due to the shorter pulse duration (3 nsec) than the thermal relaxation time of melanin (1msec). There is a high success rate in lowering the IOP with this technique as is often the first choice therapy in POAG these days to prevent a lifetime use of drops
Iridotomy- an argon laser is used to make a hole in the iris. This allows the pressure to stabilize, enabling the iris and ciliary body to lie back down normally
Trabeculectomy – Make an alternate drainage system if other methods aren’t working (diagram)
When to refer?
OPEN ANGLE
This is not always dependent on IOP. Normally we are advised to refer if 2 out of the 3 possible findings explained about are out with normal.
- IOP >21 (considering CCT)
- Visual Fields – any repeatable defect consistent with Glaucoma in either or both eyes
- Optic Nerve – any suspicious ONH changes
CLOSED ANGLE/RISK OF ANGLE CLOSURE
- Van Herick technique – the peripheral anterior chamber width is one quarter or less of the corneal thickness
- Gonioscopy – if ≥270 degrees of posterior pigmented trabecular meshwork is not visible
OCULAR HYPERTENSION
- Intraocular pressure >25 mm Hg may be considered for referral to secondary-eye-care services irrespective of central corneal thickness.
- Ocular hypertension with intraocular pressure <26 mm Hg and central corneal thickness <555 micrometers should be referred to secondary-eye-care services if they are aged ≤65.
Note – Patients who have ocular hypertension with intraocular pressure <26 mm Hg and central corneal thickness ≥555 micrometers may be monitored in the community.
SOURCES
Scottish Intercollegiate Guidelines Network (SIGN), Glaucoma referral and safe discharge. http://www.sign.ac.uk/pdf/SIGN144.pdf
J J Kanski 6th edition, Clinical Ophthalmology
Root, T Chapter 3, Glaucoma. July 20, 2009